
Chemistry, 04.12.2019 04:31 tra10money
Classify each statement as true or false. drag the appropriate items to their respective bins. view available hint(s) reset the valence electrons of group 4a elements are in the 5s subshell. period 3 elements have an inner electron configuration of [ar). the highest principal quantum number of period 4 elements is 4 period 5 elements have six 4p electrons period 5 elements have an inner electron configuration of (kr group 8a elements have full outer principal s and p subshells. the valence electrons of group 3a elements are in an s and p subshell. the highest principal quantum number of period 4 elements is 5 false true submit

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
Classify each statement as true or false. drag the appropriate items to their respective bins. view...
Questions in other subjects:










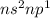 .
.

