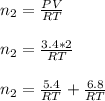Chemistry, 04.12.2019 04:31 mackwackuwu
The valve between a 2.00-l bulb, in which the gas pressure is 1.80 atm, and a 3.00-l bulb, in which the gas pressure is 3.40 atm, is opened. what is the final pressure in the two bulbs, the temperature remaining constant?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
The valve between a 2.00-l bulb, in which the gas pressure is 1.80 atm, and a 3.00-l bulb, in which...
Questions in other subjects:

Social Studies, 17.05.2021 05:10


History, 17.05.2021 05:10

World Languages, 17.05.2021 05:10

English, 17.05.2021 05:10

English, 17.05.2021 05:10



Chemistry, 17.05.2021 05:10

English, 17.05.2021 05:10