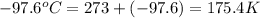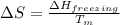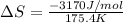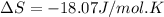Chemistry, 04.12.2019 04:31 cutebabyolivia
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing temperature (–97.6˚c) and 1 atm? report your answer two points past the decimal with the unit j/molk. ∆h˚fus = 3.17 kj/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1


Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

You know the right answer?
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing tem...
Questions in other subjects:




Mathematics, 01.11.2021 21:20



Mathematics, 01.11.2021 21:30

English, 01.11.2021 21:30


Geography, 01.11.2021 21:30

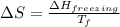
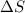 = change in entropy
= change in entropy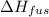 = change in enthalpy of fusion = 3.17 kJ/mol
= change in enthalpy of fusion = 3.17 kJ/mol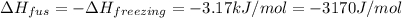
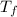 = freezing point temperature =
= freezing point temperature =