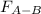Chemistry, 04.12.2019 03:31 christophercordero15
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to be a large positive number, a large negative number, or close to zero? fill in the following sentence: δhsoln is determined by the relative magnitudes of the "old" solute-solute (δhsolute) and solvent-solvent (δhsolvent) interactions and the "new" solute-solvent (δhmix) interactions. in this process, the energy of mixing hexane and heptane (δhmix) is the energy of separating them individually (δhsolute + δhsolvent), so δhsoln is expected to be .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
You know the right answer?
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to...
Questions in other subjects:


Mathematics, 08.12.2020 04:10



Biology, 08.12.2020 04:10

Mathematics, 08.12.2020 04:10

Mathematics, 08.12.2020 04:10

Mathematics, 08.12.2020 04:10

Social Studies, 08.12.2020 04:10

 =
=  =
=