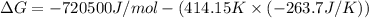Chemistry, 04.12.2019 03:31 ashcookie27
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its constituent elements,
p2(g) + 3cl2(g) > 2pcl3(g)
is kj/mol. at 25.0 degrees celsius for this reaction, delta h is -720.5 kj/mol, delta g is -642.9 kj/mol, and delta s is -263.7 j/k.
a.) -829.7
b.) 1.08 x 10^5
c.) 3.65 x 10^4
d.) -683.3
e.) -611.3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3
You know the right answer?
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its...
Questions in other subjects:



Geography, 02.08.2019 02:40

English, 02.08.2019 02:40

Health, 02.08.2019 02:40