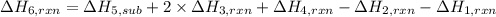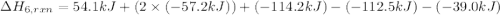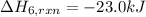Chemistry, 04.12.2019 02:31 austinparness02
The chemistry of nitrogen oxides is very versatile. given the following reactions and their standard enthalpy changes,
(1) no(g) + no
2
(g)
→
n
2
o
3
(g) ;
δ
h
o
r
x
n
= -39.kj
(2) no(g) + no
2
(g) + o
2
(g)
→
n
2
o
5
(g) ;
δ
h
o
r
x
n
= -112.5 kj
(3) 2no
2
(g)
→
n
2
o
4
(g) ;
δ
h
o
r
x
n
= -57.2 kj
(4) 2no(g) + o
2
(g)
→
2no
2
(g) ;
δ
h
o
r
x
n
= -114.2 kj
(5) n
2
o
5
(s)
→
n
2
o
5
(g) ;
δ
h
o
s
u
b
l
= 54.1 kj
calculate the heat of reaction for n
2
o
3
(g) + n
2
o
5
(s)
→
2n
2
o
4
(g)
δ
h = _ _ _ _ _ kj

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
The chemistry of nitrogen oxides is very versatile. given the following reactions and their standard...
Questions in other subjects:

Social Studies, 14.01.2021 02:20



Mathematics, 14.01.2021 02:20


Mathematics, 14.01.2021 02:20

History, 14.01.2021 02:20

German, 14.01.2021 02:20

Mathematics, 14.01.2021 02:20

French, 14.01.2021 02:20

 ..[1]
..[1] ..[2]
..[2]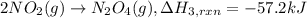 ..[3]
..[3]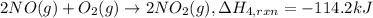 ..[4]
..[4]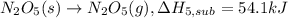 ..[5]
..[5]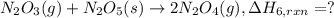 ..[6]
..[6]