
Chemistry, 04.12.2019 02:31 dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions in other subjects:

Mathematics, 29.09.2019 18:00



Mathematics, 29.09.2019 18:00

Mathematics, 29.09.2019 18:00

English, 29.09.2019 18:00

Physics, 29.09.2019 18:00

Mathematics, 29.09.2019 18:00


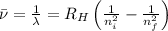
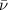 = Wave number
= Wave number = Wavelength of radiation
= Wavelength of radiation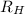 = Rydberg's Constant
= Rydberg's Constant = Higher energy level
= Higher energy level 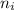 = Lower energy level
= Lower energy level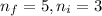

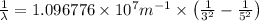
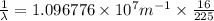
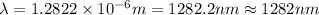
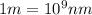 )
)

