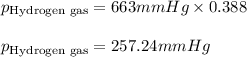Chemistry, 04.12.2019 01:31 andrejr0330jr
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of nitrogen and 0.156 grams of hydrogen. what is the partial pressure of each gas in the mixture? pn2 = mm hg ph2 = mm hg

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of ni...
Questions in other subjects:







English, 24.08.2021 04:50



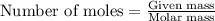
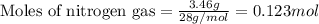
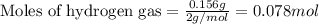
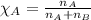 ......(1)
......(1)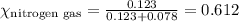
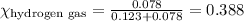
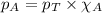 ......(2)
......(2)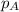 = partial pressure of substance A
= partial pressure of substance A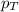 = total pressure = 663 mmHg
= total pressure = 663 mmHg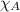 = mole fraction of substance A
= mole fraction of substance A