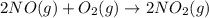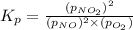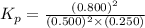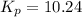Chemistry, 04.12.2019 01:31 Kingzion5775
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all species. for the reaction
2 no ( g ) + o 2 ( g ) > 2 no 2 ( g )
the standard change in gibbs free energy is δ g ° = − 32.8 kj / mol . what is δ g for this reaction at 298 k when the partial pressures are:
pno = 0.500 bar , po2 = 0.250 bar , and pno 2 = 0.800 bar
deltag = ?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all specie...
Questions in other subjects:


English, 06.05.2020 22:03




Mathematics, 06.05.2020 22:03

Social Studies, 06.05.2020 22:03



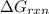 is, -27.0kJ/mole
is, -27.0kJ/mole ............(1)
............(1)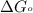 = standard Gibbs free energy = -32.8 kJ
= standard Gibbs free energy = -32.8 kJ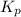 = equilibrium constant
= equilibrium constant