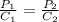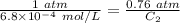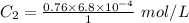Chemistry, 04.12.2019 00:31 fireman59937
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitrogen gas in air is 0.76 atm, what is the concentration (molarity) of dissolved nitrogen?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitr...
Questions in other subjects:

History, 19.04.2021 02:40

English, 19.04.2021 02:40


Mathematics, 19.04.2021 02:40


Mathematics, 19.04.2021 02:40



Mathematics, 19.04.2021 02:40

Mathematics, 19.04.2021 02:40