
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) and gaseous water (h20). what is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? be sure your answer has the correct number of significant digits in it. 02

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) an...
Questions in other subjects:


Mathematics, 10.07.2019 12:10

Mathematics, 10.07.2019 12:10



Mathematics, 10.07.2019 12:10


English, 10.07.2019 12:10


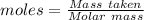
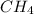 :-
:- 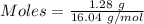
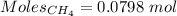
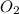 :-
:-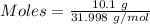
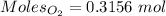
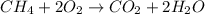
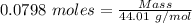
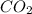 = 3.51 g
= 3.51 g

