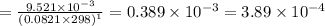Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1



Chemistry, 23.06.2019 09:30, gabriel5575
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
Consider the following equilibrium.2 nobr(g)< => 2 no(g) + br2(g)if nitrosyl bromide, nobr, i...
Questions in other subjects:



History, 21.08.2019 23:30



Mathematics, 21.08.2019 23:30




Social Studies, 21.08.2019 23:30

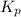 of the reaction is
of the reaction is 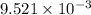 .
.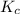 of the reaction is
of the reaction is 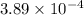 .
.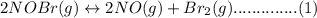
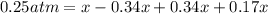
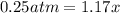
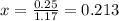
![[]P_{NOBr}]](/tpl/images/0401/8095/a6c6d.png) is 0.14 atm.
is 0.14 atm.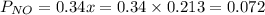
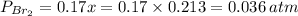
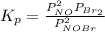
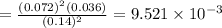
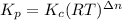
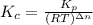
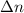 = number of moles of reactants - Number of moles of products
= number of moles of reactants - Number of moles of products