
Chemistry, 03.12.2019 22:31 ronnie7898
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0 ml of 1 m h2so4. a 25.00 ml aliquot is analyzed by adding ki and titrating the i3^- that forms with s2o3^2-. the end point was reached following the addition of 13.02 ml of 0.03247 m na2s2o3. calculate the weight percent of ce^4+ in the sample?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 19:20, Lovelybunny321
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0 ml of 1 m h2so4....
Questions in other subjects:

Social Studies, 24.09.2019 16:30



Social Studies, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30




Health, 24.09.2019 16:30

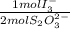 = 2,114x10⁻⁴ moles of I₃⁻
= 2,114x10⁻⁴ moles of I₃⁻ = 4,228x10⁻⁴ moles of Ce(IV).
= 4,228x10⁻⁴ moles of Ce(IV). 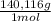 = 0,05924 g of Ce(IV)
= 0,05924 g of Ce(IV)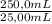 =0,5924g of Ce(IV) in the sample
=0,5924g of Ce(IV) in the sample

