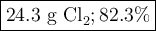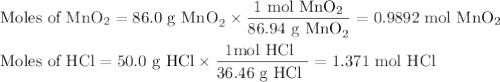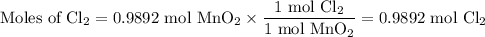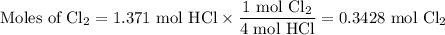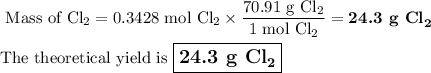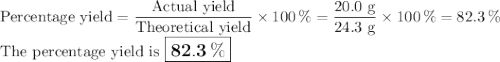Chemistry, 03.12.2019 22:31 kellynadine02
Chlorine forms from the reaction of hydrochloric acid with manganese(iv) oxide. calculate the theoretical yield and the percent of chlorine if 86.0g of mno2 and 50.0g of hcl react. the actual yield of cl2 is 20.0g.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Chlorine forms from the reaction of hydrochloric acid with manganese(iv) oxide. calculate the theore...
Questions in other subjects:

Social Studies, 04.04.2020 18:23


Mathematics, 04.04.2020 18:23


Biology, 04.04.2020 18:24



Chemistry, 04.04.2020 18:24