Chemistry, 03.12.2019 21:31 zaniathomasel
The combustion of pentane, c5h12, occurs via the reaction c5h12(g)+8o2(g)→5co2(g)+6h2o(g) with heat of formation values given by the following table: substance δh∘f (kj/mol) c5h12 (g) -119.9 co2(g) −393.5 h2o(g) −241.8 calculate the enthalpy for the combustion of pentane. express your answer to four significant figures and include the appropriate units.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
The combustion of pentane, c5h12, occurs via the reaction c5h12(g)+8o2(g)→5co2(g)+6h2o(g) with heat...
Questions in other subjects:

History, 19.11.2020 19:00


English, 19.11.2020 19:00







History, 19.11.2020 19:00

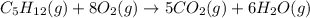
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0401/5602/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{CO_2}\times \Delta H_{CO_2})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_5H_{12}}\times \Delta H_{C_5H_{12}})]](/tpl/images/0401/5602/99eed.png)
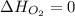 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(6\times -241.8)+(5\times -393.5)]-[(8\times 0)+(1\times -119.9)]](/tpl/images/0401/5602/0be52.png)