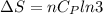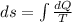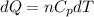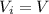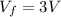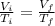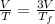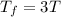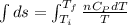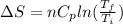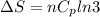Chemistry, 03.12.2019 21:31 squidmeat12
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi to volume 3vi. find the change in entropy of the gas by calculating, ∫dq / t, where dq = ncpdt. (use the following as necessary: cp and n.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi...
Questions in other subjects:





Mathematics, 15.04.2020 04:00

Mathematics, 15.04.2020 04:00