
Chemistry, 03.12.2019 21:31 courtnicollee
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii): 4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l)=4fe(oh)3(s)h ow many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the soluti...
Questions in other subjects:






Social Studies, 18.10.2019 17:30




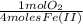 = 1,6x10⁻³ moles of O₂(g)
= 1,6x10⁻³ moles of O₂(g)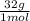 = 0,051g of O₂ are consumed
= 0,051g of O₂ are consumed


