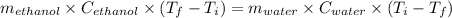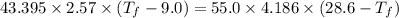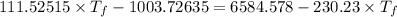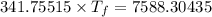Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
If 55.0 ml of ethanol (density=0.789g/ml)) initially at 9.0 ∘c is mixed with 55.0 ml of water (densi...
Questions in other subjects:

Mathematics, 01.04.2021 17:20

Physics, 01.04.2021 17:20

Mathematics, 01.04.2021 17:20

Mathematics, 01.04.2021 17:20


Mathematics, 01.04.2021 17:20


Mathematics, 01.04.2021 17:20

Mathematics, 01.04.2021 17:20

Mathematics, 01.04.2021 17:20