

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
At 25.0 ⁰c the henry's law constant for hydrogen sulfide(h2s) gas in water is 0.087 m/atm. caculate...
Questions in other subjects:

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

English, 11.09.2020 06:01

Social Studies, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

German, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01


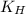 = Henry's constant =
= Henry's constant = 
 = partial pressure of hydrogen sulfide gas = 2.42 atm
= partial pressure of hydrogen sulfide gas = 2.42 atm




