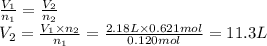Chemistry, 03.12.2019 18:31 leahstubbs
Achemical reaction occurring in a cylinder equipped with a moveable piston produces 0.621 mol of a gaseous product.
if the cylinder contained 0.120 mol of gas before the reaction and had an initial volume of 2.18 l, what was its volume after the reaction?
(assume constant pressure and temperature and that the initial amount of gas completely reacts.)

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
Achemical reaction occurring in a cylinder equipped with a moveable piston produces 0.621 mol of a g...
Questions in other subjects:




Physics, 03.02.2021 21:50

Physics, 03.02.2021 21:50

Social Studies, 03.02.2021 21:50

History, 03.02.2021 21:50



Mathematics, 03.02.2021 21:50