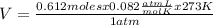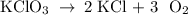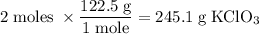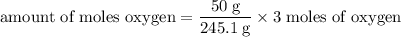Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1


Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Determine the volume of o2 (at stp) formed when 50.0 g of kclo3 decomposes according to the followin...
Questions in other subjects:


Business, 05.03.2021 16:30



Mathematics, 05.03.2021 16:30


Engineering, 05.03.2021 16:30


Geography, 05.03.2021 16:30

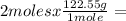 245.1 grams of KClO₃
245.1 grams of KClO₃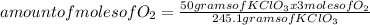
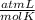 T= 0 C= 273 K
T= 0 C= 273 K