
Chemistry, 03.12.2019 18:31 taridunkley724
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of 700.0 torr. the partial pressure of water vapor at 20.00∘c is 17.5 torr. calculate the mole fraction of h2 gas in the sample.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 16:30, firstone04kr
Aresearcher wants to experiment with an element that reacts like phosphorus (p) but has a greater atomic mass. which element should the researcher select for the experiment?
Answers: 1

Chemistry, 24.06.2019 00:30, aaronolivera200161
What intermolecular forces are present in each of the substances? c5h12 , ch3oh , ch3f , ch4 , nh3 dispersion forces only, dispersion/dipole-dipole, or dispersion forces/dipole-dipole/hydrogen bonding.
Answers: 2
You know the right answer?
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of...
Questions in other subjects:



Mathematics, 13.01.2021 06:50



History, 13.01.2021 06:50


Mathematics, 13.01.2021 06:50



 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr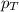 = total pressure = 700.0 torr
= total pressure = 700.0 torr = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?


