
Chemistry, 03.12.2019 17:31 alyssagibson470
A0.10 m solution of a weak monoprotic acid has a ph of 3.40 at 25°c. what is the acid-ionization
constant, ka, for this acid?
a) 1.6 x 10-6
b) 4.0 x 10-4
c) 3.4 x 10-5
d) 1.2 x 10-3
e) 1.8 x 10-7

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
A0.10 m solution of a weak monoprotic acid has a ph of 3.40 at 25°c. what is the acid-ionization
Questions in other subjects:








Computers and Technology, 30.11.2019 02:31



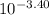 = 3.98 x 10⁻⁴
= 3.98 x 10⁻⁴


