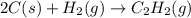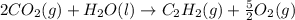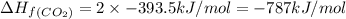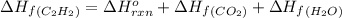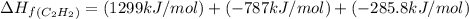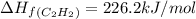The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) >...

The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) > > > > > > > co2 (g) + h2o (l) heat of reaction (rxn) = -1299kj/mol
calculate the enthalpy of formation of accetylene, given the following enthalpies of formation
standard formation [co2 (g)]= -393.5 kj/mol
standard formation [h2o (l)] = -285.8 kj/mol

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
Questions in other subjects:

History, 21.09.2021 14:00




English, 21.09.2021 14:00

Social Studies, 21.09.2021 14:00



Mathematics, 21.09.2021 14:00

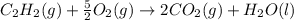
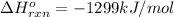
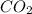 will be,
will be,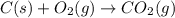

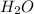 will be,
will be,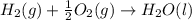
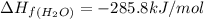
 will be,
will be,