
Chemistry, 03.12.2019 05:31 ErrorNameTaken505
The reaction of 50 ml of gas with 50 ml of gas via the equation: cl2(g) + c2h4(g) ➔ c2h4cl2 (g) will produce a total of ml of products if pressure and temperature are kept constant.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1
You know the right answer?
The reaction of 50 ml of gas with 50 ml of gas via the equation: cl2(g) + c2h4(g) ➔ c2h4cl2 (g) wil...
Questions in other subjects:




Mathematics, 19.01.2021 16:50






Mathematics, 19.01.2021 16:50

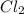 , 50 ml of
, 50 ml of 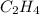
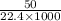 (As 1 L = 1000 ml)
(As 1 L = 1000 ml)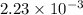 moles
moles 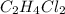 is equal to the moles of
is equal to the moles of 


