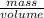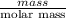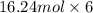Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmospheric pressure of 755 mmhg and 20.3 °c. the volume of container 1 is 2.09 l, and it contains 7.61 mol of the gas. the volume of container 2 is 4.46 l. determine the moles of f atoms in container 2 and the density of the gas at the conditions in the room

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

You know the right answer?
Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmosph...
Questions in other subjects:










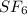 in container- 1 is as follows.
in container- 1 is as follows.