
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5.0 g of o2, and the other flask contains 5.0 g of h2. is each of the following statements true or false? explain.
a) true. because the gases have the same volumes, they must have the same number of molecules.
b) false. because the molar mass of o2 is greater than the molar mass of h2, 5.0g of o2 will contain fewer molecules than 5.0 g of h2.
c)false. depending on the pressure each flask may contain different numbers of molecules.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5...
Questions in other subjects:

Chemistry, 04.10.2019 18:30



Mathematics, 04.10.2019 18:30

History, 04.10.2019 18:30

Social Studies, 04.10.2019 18:30

Mathematics, 04.10.2019 18:30

Mathematics, 04.10.2019 18:30

History, 04.10.2019 18:30

History, 04.10.2019 18:30


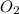 =
= 


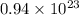 molecules
molecules =
= 
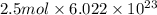
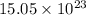 molecules
molecules![O_{2} will contain same molecules as 5.0 g of [tex]H_{2}](/tpl/images/0400/5815/5c250.png) is not true.
is not true. (at constant temperature and number of moles)
(at constant temperature and number of moles)

