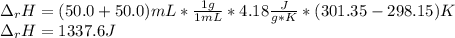Chemistry, 03.12.2019 04:31 dhananjaynagarkar
When 50.0 ml of 0.500 m hcl at 25.0°c is added to 50.0 ml of 0.500 m naoh at 25.0°c in a coffee cup calorimeter, the temperature of the mixture rises to 28.2°c.
what is the enthalpy of reaction per mole of acid?
assume the mixture has a specific heat capacity of 4.18 j/(g ? k) and that the densities of the reactant solutions are both 1.00 g/ml.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 10:00, alejandra216
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1
You know the right answer?
When 50.0 ml of 0.500 m hcl at 25.0°c is added to 50.0 ml of 0.500 m naoh at 25.0°c in a coffee cup...
Questions in other subjects:

Mathematics, 23.09.2021 23:20


Mathematics, 23.09.2021 23:20

Social Studies, 23.09.2021 23:20

Mathematics, 23.09.2021 23:30




Mathematics, 23.09.2021 23:30

Mathematics, 23.09.2021 23:30