

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, lexybellx3
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3


Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
What is the value for the reaction: n2(g) + 2 o2(g) --> n2o4(g) in terms of k values from the r...
Questions in other subjects:


Mathematics, 12.07.2019 07:00

Geography, 12.07.2019 07:00

Mathematics, 12.07.2019 07:00


Mathematics, 12.07.2019 07:00

Mathematics, 12.07.2019 07:00

Mathematics, 12.07.2019 07:00

Mathematics, 12.07.2019 07:00

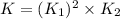
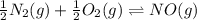
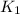
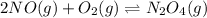
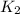
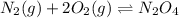
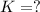
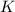 for the final reaction.
for the final reaction.

