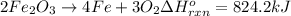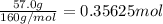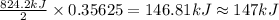Chemistry, 03.12.2019 03:31 dashavasilisk
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe + 3o2 deltah degree rxn = + 824.2 kj decomposition of 57.0 g of fe2o3 results in the release of 294 kj of heat. a. the absorption of 23500 kj of heat. b. the absorption of 147 kj of heat. c. the absorption of 294 kj of heat. d. the release of 23500 kj of heat. e. the release of 147 kj of heat.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe +...
Questions in other subjects:





History, 25.08.2020 23:01



Mathematics, 25.08.2020 23:01

History, 25.08.2020 23:01

English, 25.08.2020 23:01