consider this system: ch3cooh(aq) h+(aq) + ch3coo- ka = 1.8 x 10-5

Chemistry, 17.01.2020 07:31 adam15200031
Select all that apply.
consider this system: ch3cooh(aq) h+(aq) + ch3coo- ka = 1.8 x 10-5
what will happen to the equilibrium as oh- ions are added?
a) h+ will combine with oh- to form water.
b) [ch3cooh] will increase.
c) the acid will dissociate to supply more h+.
d) the reaction will move to the right.
e) the reaction will move to the left.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1


Chemistry, 23.06.2019 05:00, cpcoolestkid4
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius. a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit. a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius. a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Select all that apply.
consider this system: ch3cooh(aq) h+(aq) + ch3coo- ka = 1.8 x 10-5
consider this system: ch3cooh(aq) h+(aq) + ch3coo- ka = 1.8 x 10-5
Questions in other subjects:

Social Studies, 20.07.2019 01:00

Physics, 20.07.2019 01:00




Biology, 20.07.2019 01:00

Mathematics, 20.07.2019 01:00


Mathematics, 20.07.2019 01:00

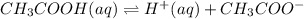
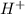 are decreased, the equilibrium will shift in a direction where concentration of
are decreased, the equilibrium will shift in a direction where concentration of 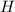 ions. During this process the reaction will move to the right direction.
ions. During this process the reaction will move to the right direction.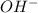 are added then the
are added then the 


