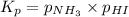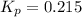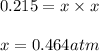Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) at 400 ºc, kp = 0.215. calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400 ºc. complete the ice box below as part of your answer.
a. 0.103 atmb. 0.215 atmc. 0.232 atmd. 0.464 atme. 2.00 atm

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

You know the right answer?
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) a...
Questions in other subjects:



Physics, 11.07.2019 02:00

English, 11.07.2019 02:00




English, 11.07.2019 02:00


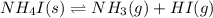
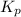 for the following equation is:
for the following equation is: