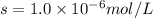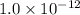agbr = 7.3 x 10-7

The molar solubilities of the following compounds (in mol/l) are:
agbr = 7.3 x 10-7
agcn = 7.7 x 10-9
agscn = 1.0 x 10-6
when these compounds are arranged in order of decreasing ksp values, what is the correct order?
agcn > agscn > agbr
agbr > agcn > agscn
agscn > agbr > agcn
agcn > agbr > agscn

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
The molar solubilities of the following compounds (in mol/l) are:
agbr = 7.3 x 10-7
agbr = 7.3 x 10-7
Questions in other subjects:






Geography, 16.10.2019 05:30




Spanish, 16.10.2019 05:30

 is
is 

![K_{sp}=[Ag^{+}][Br^-]](/tpl/images/0399/7545/d2a23.png)




![K_{sp}=[Ag^{+}][CN^-]](/tpl/images/0399/7545/d7da5.png)

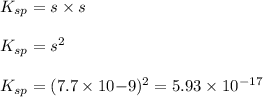
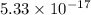

![K_{sp}=[Ag^{+}][SCN^-]](/tpl/images/0399/7545/55108.png)