
Chemistry, 02.12.2019 21:31 heatherswiffin666
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k value for the reaction: so3(g) --> so2(g) + ½ o2(g)?
k1^½
1/k1
½ k1
(1/k1)^½

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
The reaction: 2 so2(g) + o2(g) --> 2 so3(g) has an equilibrium constant of k1. what is the k va...
Questions in other subjects:



Mathematics, 13.05.2021 07:50

Biology, 13.05.2021 07:50


Mathematics, 13.05.2021 07:50





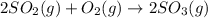

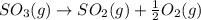
 ', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.
', the equilibrium constant of the reverse reaction will be the 1/2 power of the equilibrium constant of initial reaction.


