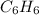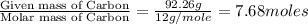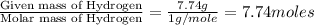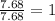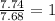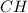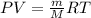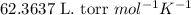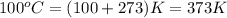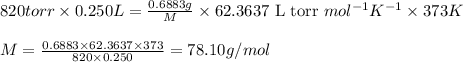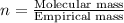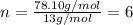Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
Acompound is 7.74% hydrogen and 92.26% carbon by mass. at 100°c a 0.6883 g sample of the gas occupie...
Questions in other subjects:

World Languages, 30.05.2020 02:57

History, 30.05.2020 02:57



Mathematics, 30.05.2020 02:57





Chemistry, 30.05.2020 02:57