Chemistry, 02.12.2019 19:31 elitehairnerd1964
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate forms and ag+ becomes negligibly small. which
of the following is the correct listing of the ions remaining in solution
in order of increasing concentration?
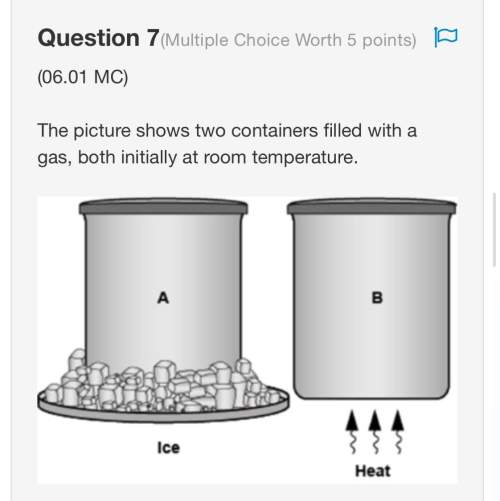
(a) po43- < no3- < na+
(b) po43- < na+ < no3-
(c) no3- < po43- < na+
(d) na+ < no3- < po43-
(e) na+ < po43- < no3-

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 21.06.2019 17:00, MichaelBoolin87241
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate fo...
a yellow precipitate fo...
Questions in other subjects:




Biology, 25.04.2021 01:00

Mathematics, 25.04.2021 01:00

Computers and Technology, 25.04.2021 01:00

Mathematics, 25.04.2021 01:00



Mathematics, 25.04.2021 01:00

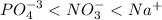
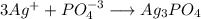
![[Ag^+]=0 M](/tpl/images/0399/5580/6ee64.png)
![[PO_4^{-3}]=0.5 M-0.5 M \frac{1 mol PO4}{3 mol Ag}=0.33 M](/tpl/images/0399/5580/8a590.png)
![[Na^+]=0.5 M * \frac{3 mol Na}{mol Na_3PO_4}=1.5 M](/tpl/images/0399/5580/07524.png)
![[NO_3^-]=0.5 M](/tpl/images/0399/5580/9ee3a.png)