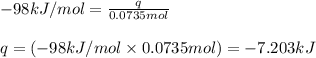Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Hydrogen peroxide decomposes to water and oxygen at constant pressure by the following reaction: 2h...
Questions in other subjects:

Mathematics, 17.09.2020 08:01

History, 17.09.2020 08:01

English, 17.09.2020 08:01

Social Studies, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Spanish, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

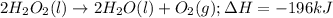
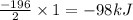
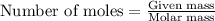
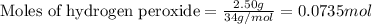
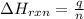
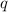 = amount of heat released
= amount of heat released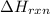 = enthalpy change of the reaction = -98 kJ/mol
= enthalpy change of the reaction = -98 kJ/mol