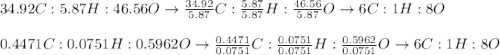Chemistry, 02.12.2019 15:31 ryleerose255
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. another sample is found to have 0.4471 g of carbon, 0.07510 g of hydrogen, and 0.5962 g of oxygen. show that these results are consistent with the law of definite proportions.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. ano...
Questions in other subjects:








Mathematics, 29.02.2020 02:03

Mathematics, 29.02.2020 02:03