
Chemistry, 01.12.2019 00:31 hei40563273
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) > rb(g) = 85.8
ie1(rb) = 397.5
be(cl2) = 226
deltahf(rbcl) = -431
electron affinity cl = -332
a. -53.7
b. +53.7
c. -695
d. -808
e. +808

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
8. select the lattice energy for rubidium chloride from the following data (in kj/mol]
rb(s) &g...
rb(s) &g...
Questions in other subjects:

Mathematics, 11.02.2021 22:00

Mathematics, 11.02.2021 22:00





Chemistry, 11.02.2021 22:00



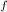 = -431 kJ/mol
= -431 kJ/mol![8. select the lattice energy for rubidium chloride from the following data (in kj/mol]rb(s) > rb](/tpl/images/0397/7697/0aca5.jpg)


