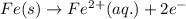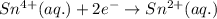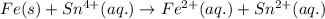Chemistry, 30.11.2019 07:31 vanessa051266
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) + fe (s) → sn2+ (aq) + fe2+ (aq) in the electrochemical cell using the redox reaction below, the anode half reaction is (aq) + (s) (aq) + (aq) fe→fe2++2e− sn4+→sn2++2e− fe+2e−→fe2+ sn4++2e−→sn2+ fe+2e−→sn2+ request answer

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1


You know the right answer?
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) +...
Questions in other subjects:

Mathematics, 01.10.2019 17:30



Mathematics, 01.10.2019 17:30

History, 01.10.2019 17:30

Chemistry, 01.10.2019 17:30




Health, 01.10.2019 17:30