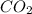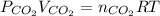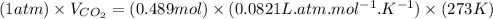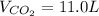Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
What volume of carbon dioxide is produced when 0.489 mol of calcium carbonate reacts completely acco...
Questions in other subjects:



Biology, 17.05.2021 16:30


History, 17.05.2021 16:30


Engineering, 17.05.2021 16:30



Mathematics, 17.05.2021 16:30

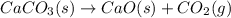
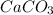 produces 1 mol of
produces 1 mol of