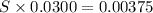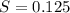What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.300 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq).

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions in other subjects:

Social Studies, 03.01.2020 21:31




Physics, 03.01.2020 21:31

English, 03.01.2020 21:31




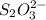 completely react with 1 mol of
completely react with 1 mol of 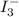 .
.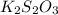 solution =
solution = 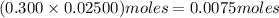
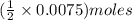 of
of 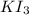 solution is S (M) then-
solution is S (M) then-