

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Calculate the amount of work done against an atmospheric pressure of 1.00 atm when 500.0 g of zinc d...
Questions in other subjects:


Mathematics, 30.03.2021 07:00



Mathematics, 30.03.2021 07:00


Mathematics, 30.03.2021 07:00


History, 30.03.2021 07:00

Mathematics, 30.03.2021 07:00

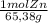 ×
×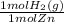 = 7,648 moles of H₂
= 7,648 moles of H₂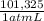 = 19,26 kJ
= 19,26 kJ

