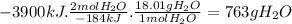Chemistry, 30.11.2019 05:31 HistoryLee
According to the following thermochemical equation, what mass of h2o (in g) must form in order to produce 3900 kj of energy?
sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj 216 g 382 g 763 g 408 g 272 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
According to the following thermochemical equation, what mass of h2o (in g) must form in order to pr...
Questions in other subjects:

Mathematics, 01.04.2021 01:00

Chemistry, 01.04.2021 01:00








Mathematics, 01.04.2021 01:00