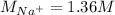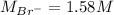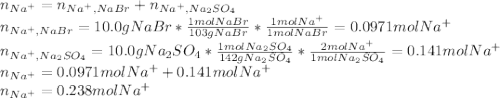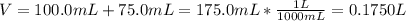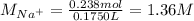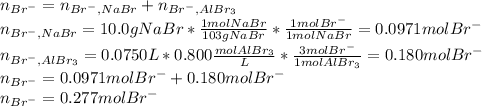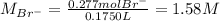Chemistry, 30.11.2019 03:31 caplode7497
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml solution. this solution is then mixed with 75.0 ml of a 0.800 m aqueous solution of albr3. calculate the concentration (m) of na+ and br− in the final solution.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml...
Questions in other subjects:



History, 13.09.2019 22:10


Social Studies, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10

History, 13.09.2019 22:10

Chemistry, 13.09.2019 22:10


Mathematics, 13.09.2019 22:10