
Chemistry, 30.11.2019 03:31 georgesarkes12
Enter a balanced equation for the reaction between aqueous lead(ii) nitrite and aqueous potassium bromide to form solid lead(ii) bromide and aqueous potassium nitrite. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
Enter a balanced equation for the reaction between aqueous lead(ii) nitrite and aqueous potassium br...
Questions in other subjects:



History, 24.02.2020 16:54




English, 24.02.2020 16:54




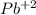 since oxidation number of lead is 2 as indicated lead (II)nitrite. The number in parentheses indicates the oxidation number.
since oxidation number of lead is 2 as indicated lead (II)nitrite. The number in parentheses indicates the oxidation number.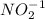 bears the charge -1.
bears the charge -1. 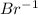 , it is a halogen and bears the charge -1.
, it is a halogen and bears the charge -1.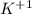 , it is an alkali metal.
, it is an alkali metal.


