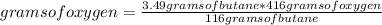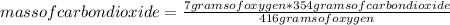Chemistry, 30.11.2019 03:31 fespinoza019
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. suppose 3.49 g of butane is mixed with 7.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, kelly3215
If a certain battery supplies 1.0×108 electrons per second to the negative terminal and the battery contains 7.00 moles of electrolytic solution (which means the solution contains 7.00 moles of hso4), what fraction of the solution undergoes a chemical reaction each second
Answers: 1

Chemistry, 21.06.2019 17:30, KindaSmartPersonn
What is the formula for the molecular compound nitrogen monoxide
Answers: 1


Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. s...
Questions in other subjects:

Mathematics, 01.02.2021 05:50

Mathematics, 01.02.2021 05:50




Mathematics, 01.02.2021 05:50

Physics, 01.02.2021 05:50

Physics, 01.02.2021 05:50

Mathematics, 01.02.2021 05:50

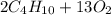 ⇒
⇒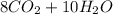
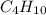 : 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol
: 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol