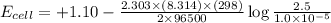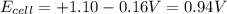The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this reaction is v when the concentration of [cu2+]=1.0⋅10−5m and [zn2+]=2.5m. zn (s) + cu2+ (aq) → cu (s) + zn2+ (aq) the standard cell potential () for the reaction below is . the cell potential for this reaction is when the concentration of and (s) + (aq) (s) + (aq) 0.78 1.10 0.94 1.26 1.42

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this r...
Questions in other subjects:


English, 03.10.2020 01:01








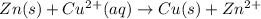
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Cu^{2+}]}](/tpl/images/0396/8274/2e6f5.png)

 = standard electrode potential of the cell = +1.10 V
= standard electrode potential of the cell = +1.10 V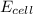 = emf of the cell = ?
= emf of the cell = ?