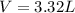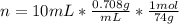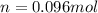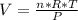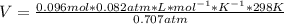Chemistry, 30.11.2019 02:31 Sparkleskeepsgoing
Diethyl ether, c4h10o was a widely used anesthetic in the early days of surgery. it has a vapor pressure of 537 torr at 25 oc. a 10.00 ml sample (d = 0.708 g/ml) is placed in a sealed 0.205 l flask. what is the maximum volume in liters the flask can have if equilibrium is to be maintained between liquid and vapor?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Diethyl ether, c4h10o was a widely used anesthetic in the early days of surgery. it has a vapor pres...
Questions in other subjects:




Health, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01

Chemistry, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01



Spanish, 28.08.2020 21:01