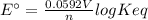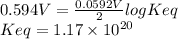Chemistry, 30.11.2019 02:31 mhuerta71001
Interactive activity—the relationship among e°cell, keq, and gibbs free energy in an electrochemical cell, the potential difference between two electrodes under standard conditions is known as the standard cell potential (e∘cell). the standard cell potential can be used to identify the overall tendency of a redox reaction to occur spontaneously. the spontaneity of a reaction is identified using the gibbs free energy δg∘. δg∘ is related to e∘cell. e∘cell and δg∘ are also related to equilibrium constant keq of the reaction. select the image to explore the activity that shows how e∘cell, keq, and δg∘ are related to each other. launch activity in the activity, you should see a triangle, whose three vertices represent keq, e∘cell, and δg∘. you can select two vertices and determine the relation between them. you can then reset the activity and select the next two quantities. constants the following values may be useful when solving this tutorial. constant value e∘cu 0.337 v e∘ni -0.257 v r 8.314 j⋅mol−1⋅k−1 f 96,485 c/mol t 298 k part a in the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are cu2+(aq)+2e−→cu(s) and ni(s)→ni2+(aq)+2e− the net reaction is cu2+(aq)+ni(s)→cu(s)+ni2+(aq) use the given standard reduction potentials in your calculation as appropriate. express your answer numerically to three significant figures.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Interactive activity—the relationship among e°cell, keq, and gibbs free energy in an electrochemical...
Questions in other subjects:







English, 26.06.2019 23:00



Mathematics, 26.06.2019 23:00